- About Us
- Services & Solutions
- Technologies
- Discovery
- Reagent Materials Generation
- Monoclonal Antibody Discovery
- Bispecific & Multispecific Antibody Generation
- Lead Optimization
- In Vitro Lead Characterization
- In Vivo Lead Characterization
- Other Molecular Modalities
- IND Filing Support
- Protein Sciences
- Antibody Production
- Protein Production
- Protein Characterization
- Protein Sciences
- Mammalian
- Microbial
- mRNA
- Viral Vaccines
- WuXi XDC – Bioconjugation
- Testing
- Sustainability
- Careers
- Investors
- News & Resources
WuXi Biologics
Offering End-to-End Solutions
Quality Assurance
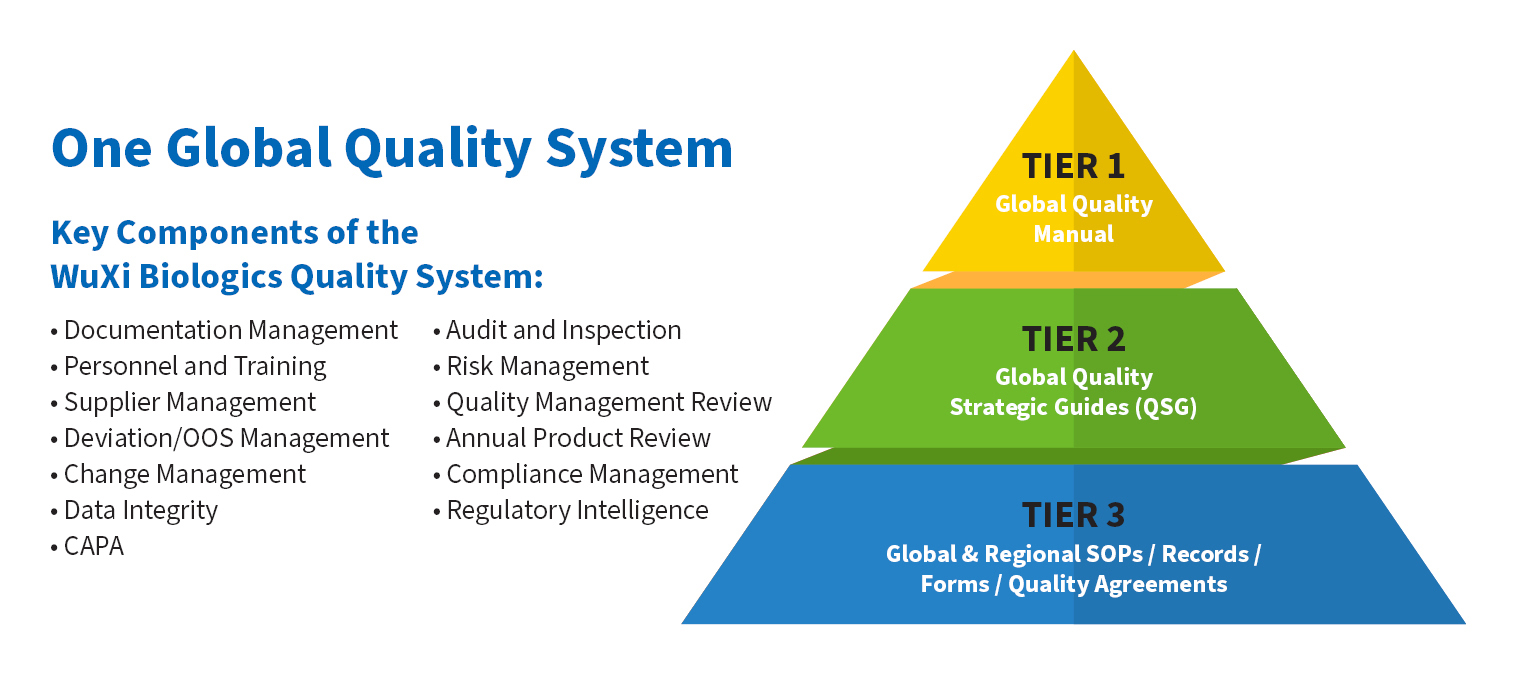
We have established a global quality system, quality control and operational systems that meet or exceed worldwide regulatory standards from the U.S. FDA, EMA, NMPA and other major regulatory agencies. Starting with our Chief Executive Officer, our commitment to quality is ingrained in our company culture and our employees and we have harmonized our quality assurance (QA) and quality management system across all sites around the world for the clinical or commercial production of biologics and vaccines drug substance and drug product.
Our commitment to quality is one of our pillars of success for enabling our clients to bring novel biotherapeutics and vaccines into the clinic and beyond. To learn more, see our video outlining our premier, world-class quality system.
Our Quality Track Record
All of our GMP manufacturing operations adhere to global regulations and guidelines including those from the FDA, EMA and NMPA. We currently have 15 different drug substance and drug product manufacturing facilities and quality control testing laboratories that have received GMP certifications.
We have passed over 1000 client GMP / quality audits and continue to strive and meet their stringent quality expectations.
We have passed 33 inspections from multiple global regulatory agencies including the U.S. FDA, EMA, NMPA, PMDA, MFDS, HSA, ANIVSA, HPRA and Health Canada.
We have enabled our clients to successfully obtain 48 product license approvals and EUAs related to BLA / MAA / EUA filings for over 10 products and from over 21 different regulatory agencies.
Using our integrated DNA to IND biologics development and manufacturing platforms, we have successfully helped our clients from all around the world to successfully file over 480 Investigational New Drug (IND) applications since 2015.
Keys to Meeting Client Quality Assurance Expectations
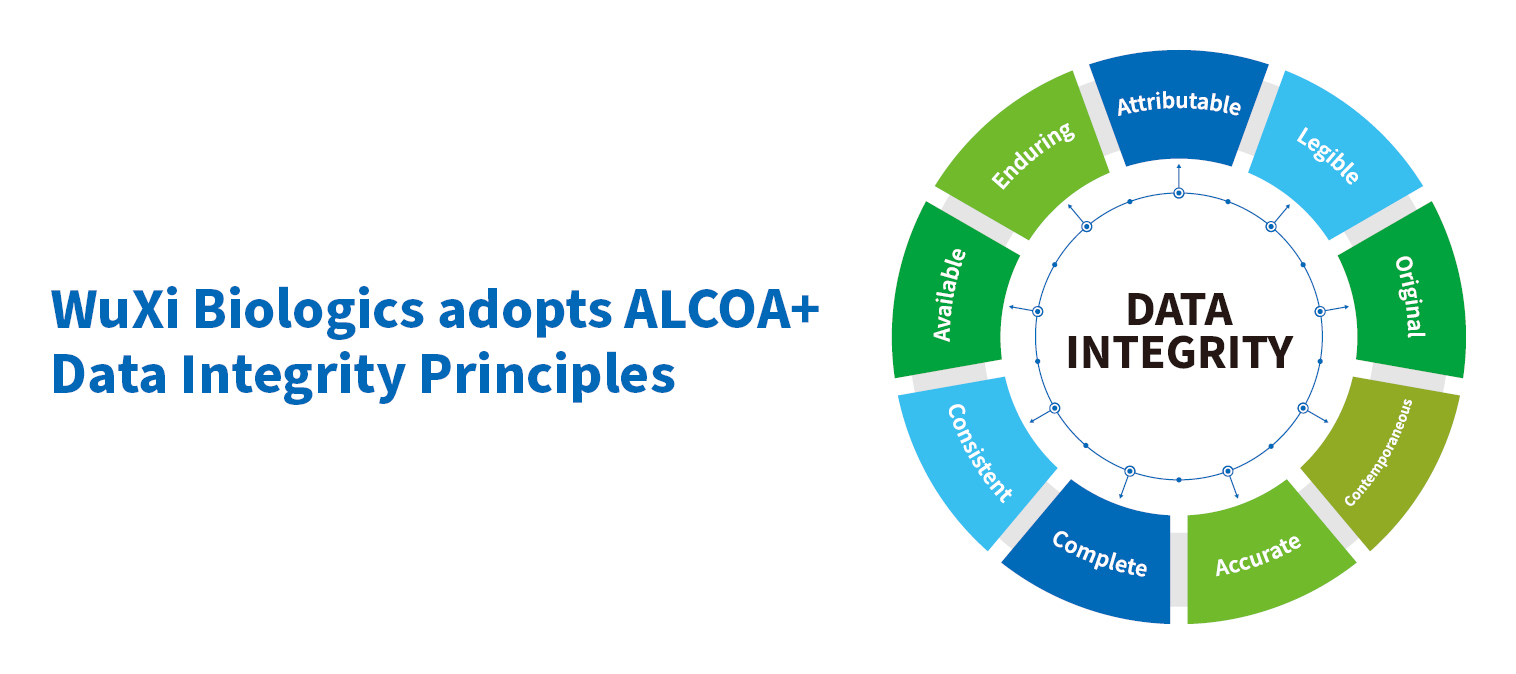
WuXi Biologics utilize ALCOA+ data integrity principles, extensive employee training programs and an unwavering commitment to protecting our client’s IP as key components in our ability to provide a premier, world-class quality system that will enable our clients to provide novel biologics and vaccines for the benefit of patients worldwide.


Protecting our clients IP is a cornerstone of our quality programs and company culture.







