- About Us
- Services & Solutions
- Technologies
- Discovery
- Reagent Materials Generation
- Monoclonal Antibody Discovery
- Bispecific & Multispecific Antibody Generation
- Lead Optimization
- In Vitro Lead Characterization
- In Vivo Lead Characterization
- Other Molecular Modalities
- IND Filing Support
- Protein Sciences
- Antibody Production
- Protein Production
- Protein Characterization
- Protein Sciences
- Mammalian
- Microbial
- mRNA
- Viral Vaccines
- WuXi XDC – Bioconjugation
- Testing
- Sustainability
- Careers
- Investors
- News & Resources
WuXi Biologics
Offering End-to-End Solutions
Analytical Development
At WuXi Biologics, our analytical development teams facilitate our one-stop mammalian cell culture-derived biologics R&D endeavors in collaboration with our other discovery and CMC development laboratories. We offer comprehensive, analytical services for a wide variety of biotherapeutics (e.g., monoclonal, bispecific and multispecific antibodies, fusion proteins, enzymes and other recombinant proteins).
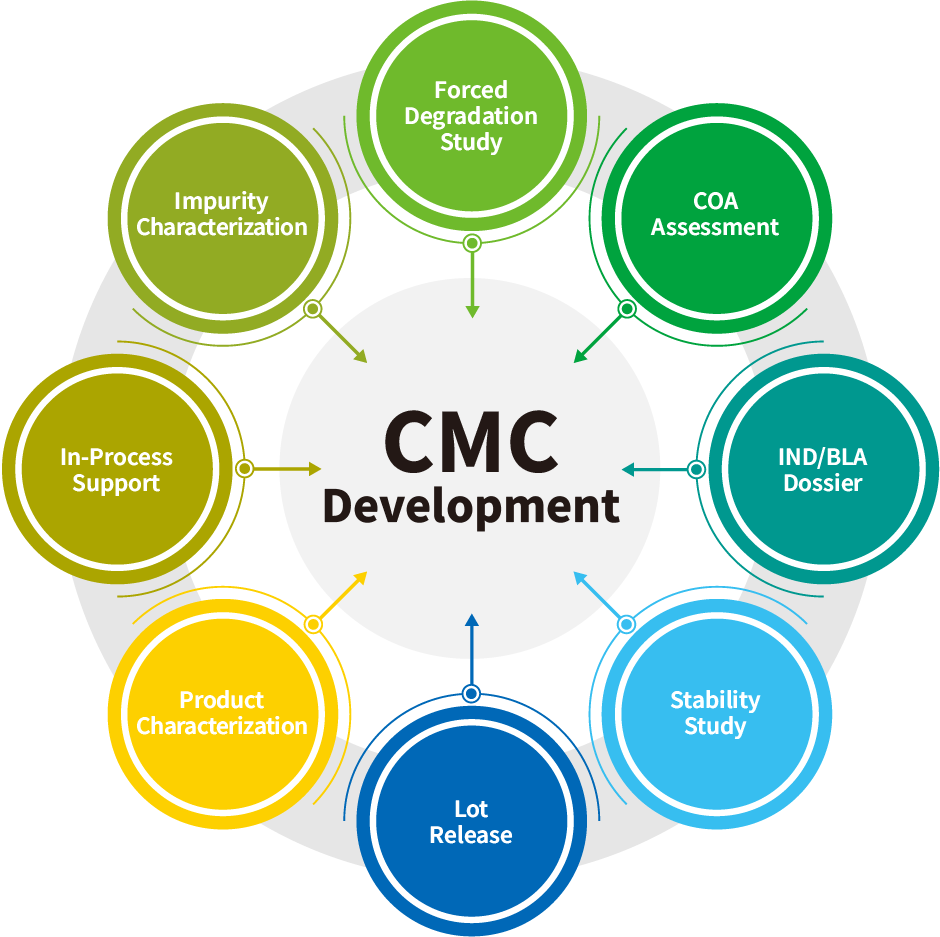
Our extensive testing services include support of developability studies, in-process analysis, cell line, process and drug product / formulation development, product characterization, lot release, stability studies and IND/BLA/MAA/EUA dossier preparation.
Our vast repertoire of analytical techniques provides the full-range of methods required for biomolecular analysis. Over 1,000 established methods including compendial, physicochemical, biochemical, biophysical and biological assays (bioassays) are available. Our highly experienced analytical team supports more than 300 integrated discovery and CMC development projects each year, generating bioanalytical data on a phenomenal scale while also providing investigation and troubleshooting support. From new drug discovery to new drug application, we manage a method’s lifecycle from development to qualification/validation and transfer to Quality Control or other departments as needed. Equipped with cutting-edge instrumentation and first-class talents, we provide systematic solutions for integrated projects while catering to special analytical needs in the form of standalone projects.
Comprehensive analytical services to support CMC Development
Additional Information:
- For more information on our extensive analytical development services – CLICK HERE
- See our analytical “Centers of Excellence”
- To capture a glimpse of how our analytical development teams support our other services see our “Case Studies for Development Services” and “Case Studies for Discovery Services”
- To learn more about our one-stop biologics development and manufacturing solutions – CLICK HERE or see our video series “The WuXi Biologics Difference”







