- Services & Solutions
- Technologies
- Discovery
- Reagent Materials Generation
- Monoclonal Antibody Discovery
- Bispecific & Multispecific Antibody Generation
- Lead Optimization
- In Vitro Lead Characterization
- In Vivo Lead Characterization
- Other Molecular Modalities
- IND Filing Support
- Protein Sciences
- Antibody Production
- Protein Production
- Protein Characterization
- Protein Sciences
- Mammalian
- Microbial
- mRNA
- Viral Vaccines
- WuXi XDC – Bioconjugation
- Testing
- News & Resources
WuXi Biologics
Offering End-to-End Solutions
>
In Vitro Lead Characterization
WuXi Biologics provides assay development services and offers comprehensive biologics screening and characterization throughout the lead preclinical candidate (PCC) drug selection process. Our biology team has extensive discovery experience with various targets and therapeutic modalities including but not limited to monoclonal (mAbs), bispecific (bsAbs) and multispecific (msAbs) antibodies, fusion proteins, and immunocytokines. Learn more about our in vitro characterization capabilities for ADCs.
Binding
Affinity by SPR (Surface Plasmon Resonance)
- Protein-Protein, protein-peptide, protein-small molecule binding affinity
- Antibody and Fc-fusion protein binding affinity to Fc receptors
- Off-rate ranking
- High-performance koff ranking for humanization and affinity maturation
- High-throughput koff ranking for hybridoma screening
Bispecific antibody simultaneous binding
- Affinity to cell surface proteins by FACS
Related SPR service
- Epitope binning
- SPR assay method development and qualification (specificity, linearity, range, accuracy, and precision)
Epitope Binning and Mapping
Peptide mapping, domain mapping and alanine scanning mutagenesis services are available to assist customers in obtaining antigen epitope information, including linear and conformational epitopes. We have successfully completed more than 30 epitope mapping projects for the benefit of our clients’ antibody drug design & discovery projects.
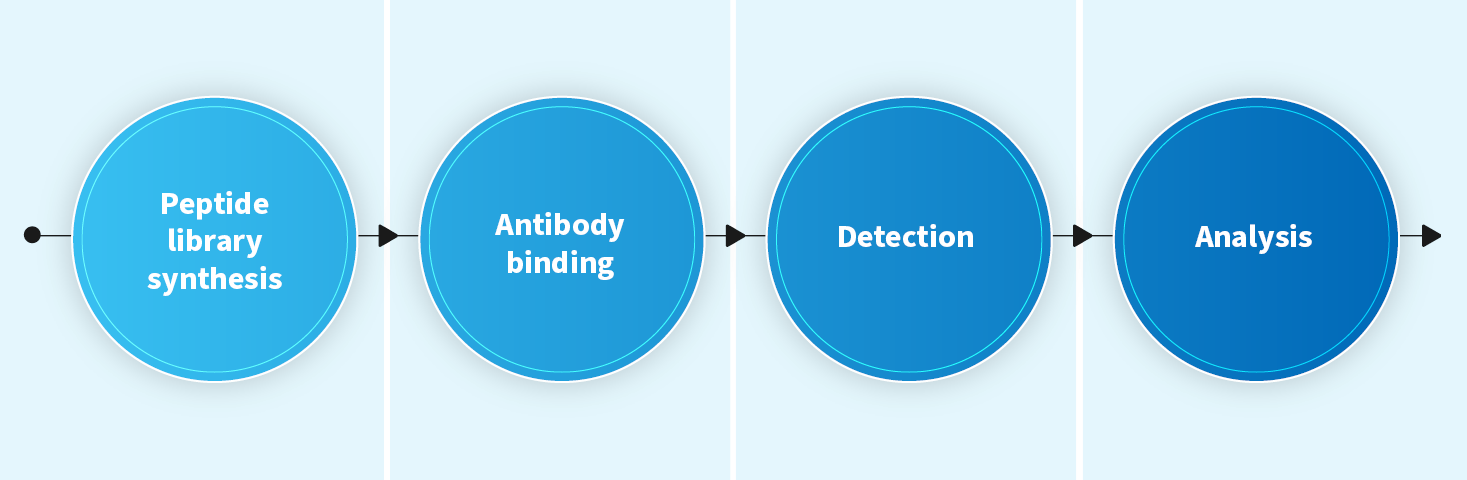
Peptide mapping (linear epitope)
- Mapping linear epitopes of a target protein
- Screening antibodies that bind to a specific epitope
- Timeline: 5~7 weeks
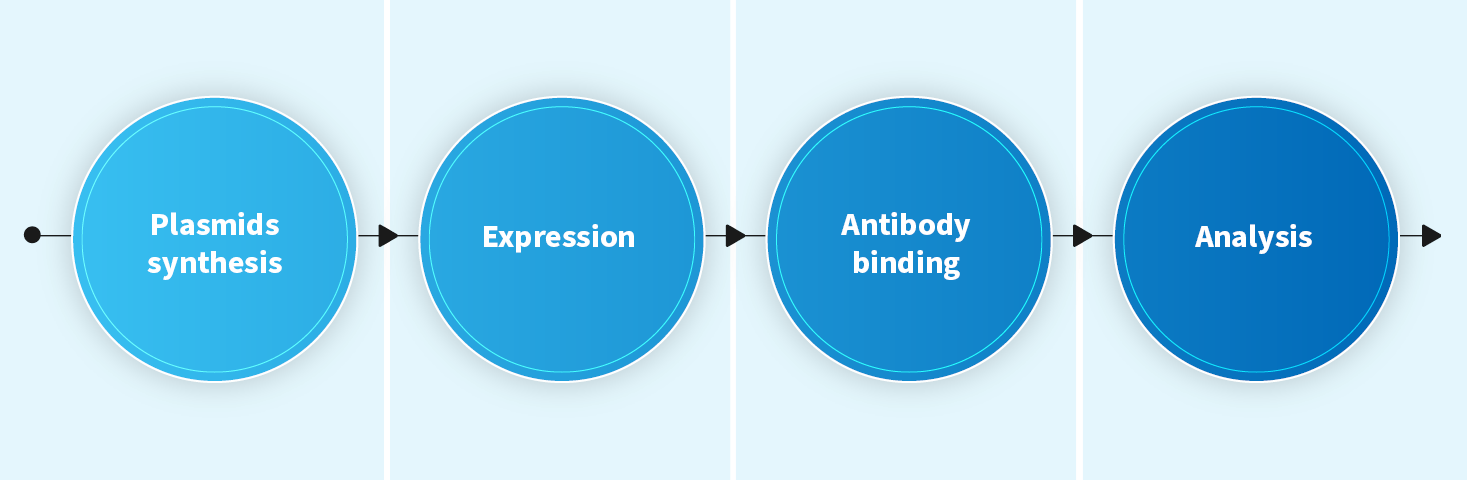
Domain mapping (domain epitope)
- Positioning the binding domain of an antibody, especially for complex, high molecular weight antigens.
- Classifying antibodies by differences in antigen-binding domains.
- Timeline: 5~7 weeks
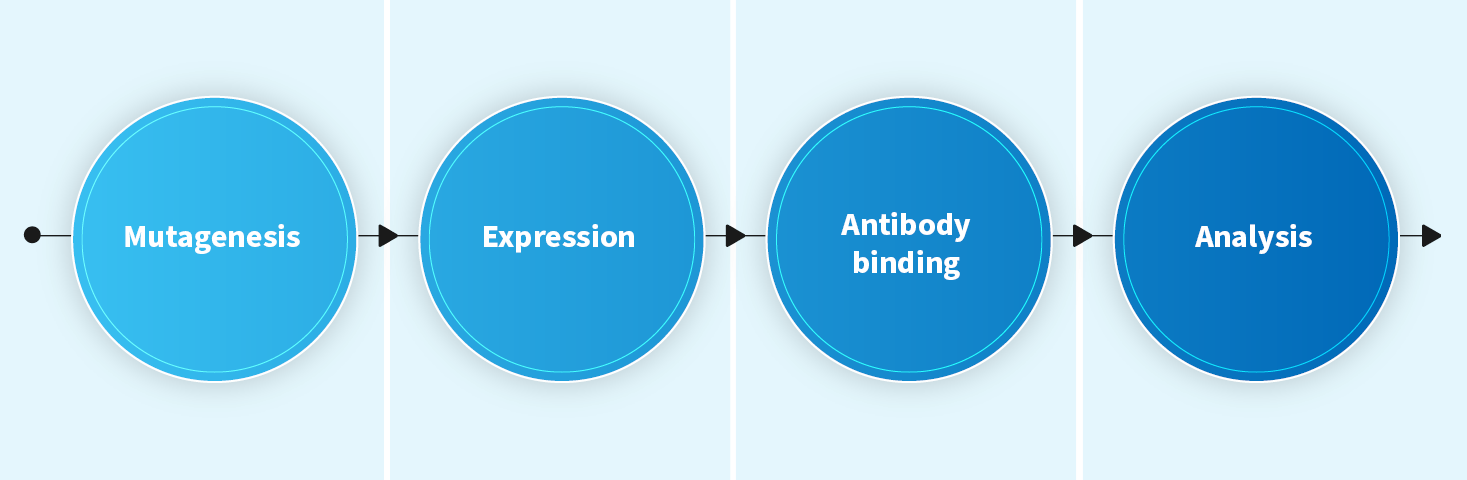
Alanine Scanning Mutagenesis (both linear and conformational epitopes)
- Mapping epitopes at the amino acid level
- Characterization of protein stability and expression
- Timeline: 9-11 weeks
Functional Assays
Biochemical Assays
| Test item | Analysis methods |
| Antigen-antibody binding assay |
|
| Competition Assay |
|
| Cross species/paralogue binding (protein or cell-based, ELISA, FACS) | |
| Signal Transduction (Phosphorylation) |
|
| cAMP signaling assay | |
| Enzymatic assays | |
| Receptor occupancy | |
Cellular Assays
| Test item | Analysis methods |
| Reporter gene assay (RGA) |
|
| Translocation imaging assay | |
| PBMC activation |
|
| T/NK/B cell activation and expansion | |
| Tumor dependent immune system activation |
|
| Fc dependent killing and phagocytosis assay |
|
| Fc independent killing |
|
| Cytotoxicity assay (CellTiter-Glo) | |
| Bystander killing assay | |
| Proliferation assay (CFSE/BrdU/CellTiter-Glo) | |
| Cell panel screening (Viability/proliferation) | |
| 3D culture assay (Proliferation, killing) | |
| Cell cycle analysis | |
| Apoptosis assay |
|
| Monocyte survival | |
| Differentiation and polarization |
|
| Antibody Internalization |
|
| Cell Migration/Invasion Assay |
|
| Surface marker profiling | |
| Target expression profiling on resting or activated cells | |
| Cytokine/chemokine profiling |
|
| Ex vivo tumor and TIL profiling | |
| CAR-T cell activation |
|
| CAR-T cell killing | |
| Allogeneic Mixed Lymphocytes Reaction (MLR) | |
| Antigen specific MLR/T cell recall assay (CMV, TT) | |
| T cell exhaustion | |
| Regulatory T cell assay |
|
| Hemagglutination | |
| Metabolic diseases | |
| Infectious disease |
|
| In vitro safety evaluation |
|
| Developability evaluation |
|
For more information on our lead characterization services please Contact Us.







