Assay Qualifications
5,300 +
Assay Validations
370 +
WuXi Biologics
Offering End-to-End Solutions
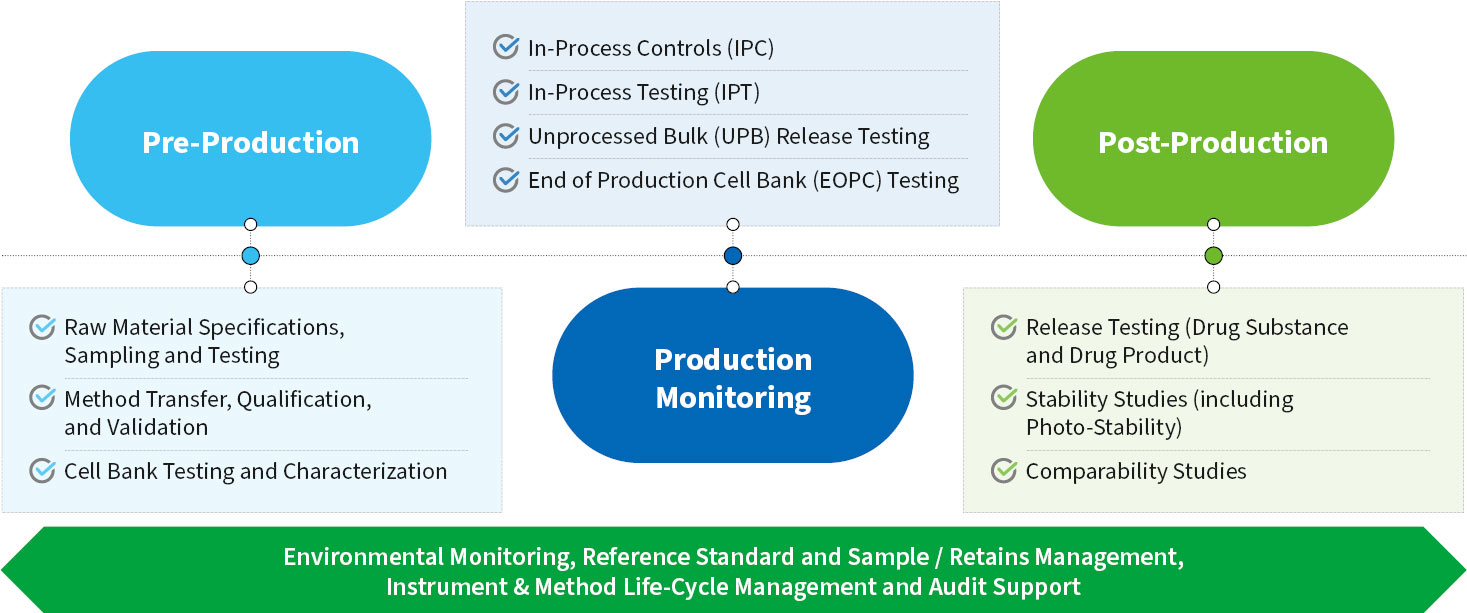
Our Quality Control (QC) labs support all WuXi Biologics clinical and commercial product GMP manufacturing sites throughout the world and work closely with manufacturing operations, Manufacturing Science and Technology (MSAT), Quality Assurance (QA), and Analytical Development teams to provide our customers with the highest quality testing services. QC oversight and activities cover pre-production (e.g., raw materials and cell bank testing), in-process control / testing and post-production functions (e.g., drug substance and drug product release, and stability and comparability studies) along with critical environmental monitoring, cleaning validation support, reference standard and sample / retains management, instrument life-cycle management and audit support functions.
Global QC maintains multiple testing Centers of Excellence including physicochemistry, biochemistry, biosafety, bioassay, microbiology, and molecular biology labs along with dedicated raw materials and environmental monitoring functions. Utilizing state-of-the-art instruments, that benchmark industry standards and electronic/software systems that includes a comprehensive and robust Laboratory Information Management System (LIMS), and other key data systems including Empower, EMC, MasterControl, and TrackWise. Other platforms, such as Lab Execution System (LES), CISPro inventory management and “elogbook” systems are utilized at specific sites. Our QC teams provide the expertise and capacity to support our client’s manufacturing programs and to perform stand-alone testing services for a wide array of biologics and vaccines. We work, as needed, alongside our large, highly trained and experienced analytical development teams to develop customized lot release and stability testing programs designed to meet regulatory guidelines and GMP regulations.
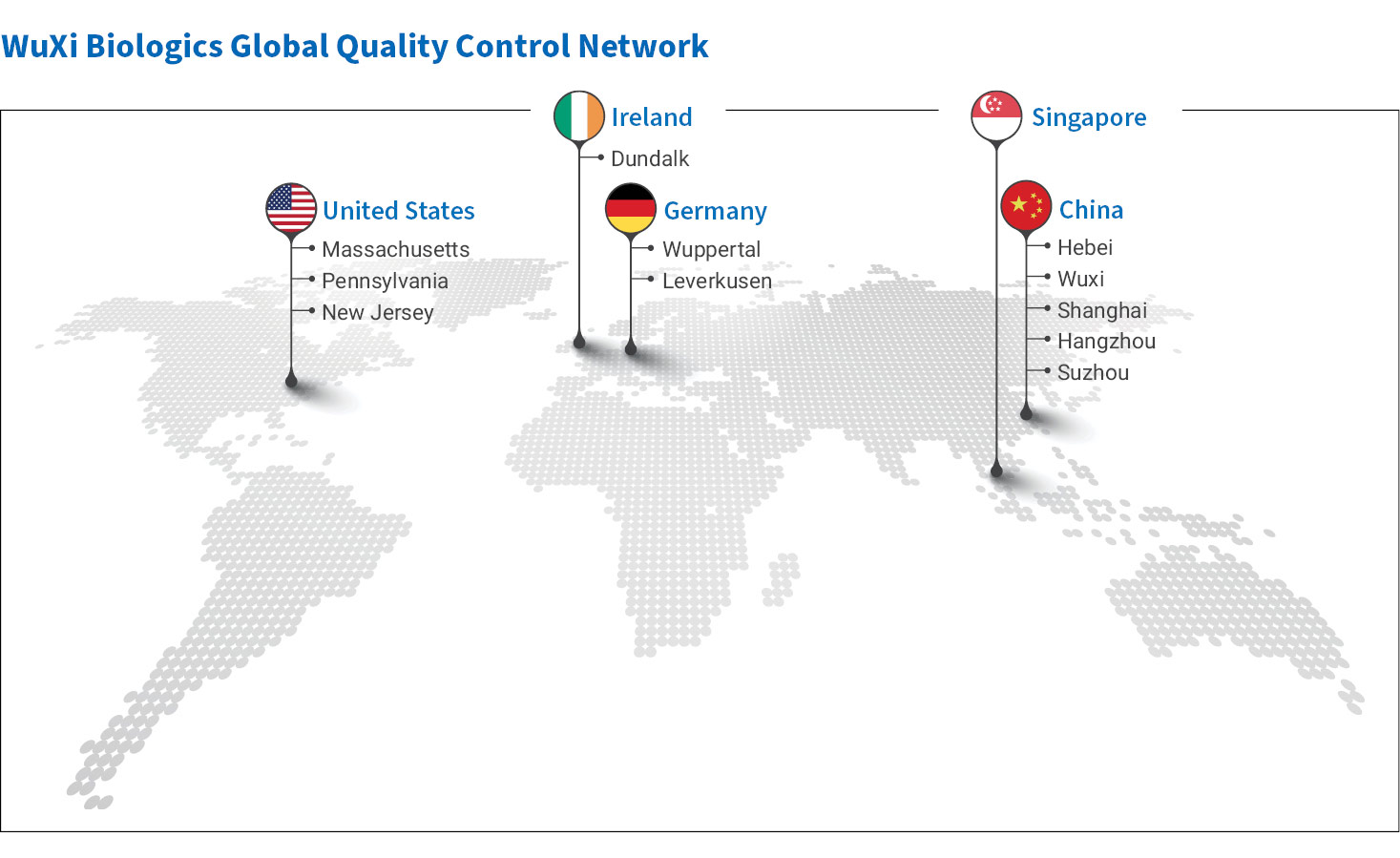
We maintain over 26,000 m2 of specific and dedicated laboratories and various functional QC Centers of Excellence to support GMP manufacturing operations and other critical quality functions within the organization. Our over 1,000 highly trained laboratory scientists and engineers around the globe provide 24/7 support to help ensure your projects stay on track so that we can meet your critical project milestones. Our network of in-house laboratories include:
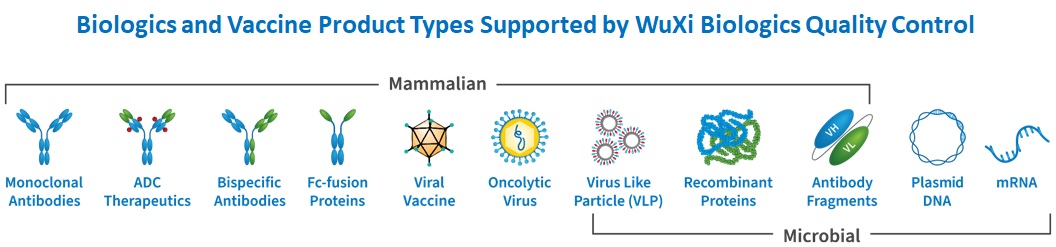
These laboratories support a wide array of biologics (e.g., monoclonal, bispecific and multispecific antibodies, fusion proteins, enzymes and other recombinant proteins) and vaccine modalities (viral, protein subunit, and nucleic acid/RNA).
QC supports pre-production preparation by ensuring the cell banks, raw materials, analytical methods, equipment and facility environment are qualified and meet the necessary quality standards.
Quality control supports analytical development teams with technical transfer and assay verification when applicable and provides phase appropriate assay specification setting, qualification and validation activities for in-process / in-line, release and stability methods. We have performed over 5,300 early-stage method qualifications and conducted over 370 late-stage method validations since beginning QC operations.
Our dedicated team supports the setting of cell bank specifications and coordinates the testing and release of over 160 cell banks (e.g., Master Cell Banks (MCB), Manufacturer’s Working Cell Banks (MWCB / WCB) and End of Production Cell Banks (EOPCs)) on an annual basis. These cell banks are used in the production of a wide array of biologics and vaccines. Release testing includes testing for adventitious agents, identity and growth characteristics per the guidelines established by the ICH and other global regulatory agencies. For more information on our comprehensive biosafety testing services – click here.
Our Raw Materials (RM) controls and testing team supports over 3,400 lots annually. These comprehensive in-house RM labs test a wide array of raw materials such as chemicals, buffers, excipients, media, supplements, resins and packaging material. The team conducts complete specifications setting, testing and release of materials used for clinical and commercial manufacturing.
QC supports the establishment and validation of in-process controls and provides the requisite in-process testing and monitoring for a variety of biologics and vaccines to ensure the production runs are conducted smoothly, efficiently and that product quality and safety standards are met.
To help ensure consistent GMP manufacture, reduce variability and identify problems during production runs our QC teams support the establishment, testing and validation (when relevant) of in-process controls. Our QC testing teams work 7 days a week, 24 hours a day to perform real-time monitoring of the various manufacturing processes to determine if in-process samples meet the established in-process control specifications. This critical function helps ensure product consistency, reliability, quality, safety, and efficacy.
WuXi Biologics analytical and biosafety testing laboratories can perform in-house all the testing required from the cell harvest or unprocessed bulk (UPB) material generated within bioreactors during biologics manufacturing including the critical adventitious agents testing (for more information on our biosafety testing capabilities – click here). We test over 1,000 UPB lots per year under GMP conditions.
QC provides post-production management of Drug substance (DS) and Drug Product (DP) lot release and stability studies (click here to see common examples of release and stability methods). As necessary, QC supports GMP operations and development teams in the testing of samples generated during comparability studies and late-stage conformance production runs.
Regardless the type of biologic or vaccine, and utilizing an extensive network of analytical, bioassay, biosafety, microbiology, molecular biology, physicochemistry and immunology laboratories our QC teams have extensive experience in the support of assay transfer, qualification and validation and routine batch testing. Our in-house, state-of-the-art labs perform release testing for over 1,000 clinical and commercial DS and DP lots annually under GMP conditions.
We provide a protocol-driven stability program integrated with our LIMS platform for scheduling stability pull-points, sample testing and tracking. Over 400 stability lots are managed by QC annually. We maintain a vast number of storage units and facilities and all stability storage settings (temperature and humidity) are maintained per ICH guidelines. Photostability stability programs are also available at multiple sites around the globe.
QC supports analytical development and GMP operations in the performance of comparability studies that are conducted to understand the impact on the quality and safety of the drug substance or drug product resulting from a change in raw materials or the manufacturing process. Similarly, QC tests samples to verify the product is of the same quality and consistency from batch to batch during late-stage process validation and conformance batch runs. Additionally, these critical studies, including post-market studies, provide evidence that the product properties remain unchanged throughout the drug‘s shelf life.
Our comprehensive quality control functions and systems are fully integrated into our GMP operations and global quality system.
Purpose built to support our global QC functions, our LIMS platform begins in the warehouse with raw material receipt and integrates all downstream functions throughout testing of raw materials, EM and in-process samples, controls/reference standards, release and stability of drug substance and drug product and final report or Certificate of Analysis (CoA) review. Our LIMS system has withstood scrutiny from over 25 regulatory agency inspections and 800+ client quality audits.
In addition to the LIMS, other electronic/software platforms, such as CISPro inventory management and Laboratory Execution System (LES) are also utilized in some sites.
We routinely perform method transfers, verifications and qualifications for every client project. We then provide late-stage method validation and maintain a comprehensive method life-cycle management program for the validated assays. The life-cycle management program includes trend analysis on assay performance to ensure robustness for all QC-related assays and methods.
Our Environment and Utility Monitoring (EM) performs routine environmental monitoring in-house for all GMP Cell Banking, Drug Substance (DS), Drug Product (DP) and QC laboratories that includes surface, air, process gas and water system samples. We perform routine EM of our >100,000 m2 clean rooms and zones in our sites globally. Routine analysis includes testing for surface microbes, settling microbes, airborne microbes and airborne particles.
QC supports IQ/OQ/PQ, calibration and maintenance aspects of the quality assurance equipment life-cycle management (ELCM) program and provides ongoing equipment / instrument monitoring to ensure all equipment used in the QC laboratories remain accurate, reliable, consistent and safe and functioning per its intended use. In addition, most QC equipment is harmonized with our analytical development labs for efficient assay transfer into quality control.
WuXi Biologics’ global quality function hosts many quality system inspections per year from either regulatory agencies (e.g., U.S. FDA, EMA, NMPA, etc.), Quality Persons or our client’s compliance / audit teams. QC supports our quality unit in all inspections / audits and together we have successfully passed over 25 regulatory inspections from multiple different global regulatory agencies and over 800 client quality audits since establishing our quality operations in 2014. We use ALCOA data integrity principles and to date, there have been no findings regarding data integrity at any of our sites around the world.
QC works closely with Quality Assurance and GMP Operations to provide ongoing management and storage of all raw materials, in-process and production batch testing samples and retains involved with or generated during GMP manufacturing. All sample / retain samples are stored per established specifications in qualified or validated storage units as applicable. The comprehensive sample / retain storage and disposal procedures are reviewed with our clients and communication procedures are reviewed periodically with current and past clients prior to disposal of any samples / retains generated in client projects. Typical storage conditions available include:
-80°C ± 10°C; -70°C ± 10°C; -60°C ± 10°C; -40°C ± 5°C; -20°C ± 5°C; 5°C ± 3°C
We offer well-established reference standard (RS) characterization and test methods and RS program management that includes reference standard generation, qualification/requalification, storage and inventory tracking and reference standard quality monitoring, stability and trending on a routine basis for over 400 reference standards on an annual basis.