- About Us
- Services & Solutions
- Technologies
- Discovery
- Reagent Materials Generation
- Monoclonal Antibody Discovery
- Bispecific & Multispecific Antibody Generation
- Lead Optimization
- In Vitro Lead Characterization
- In Vivo Lead Characterization
- Other Molecular Modalities
- IND Filing Support
- Protein Sciences
- Antibody Production
- Protein Production
- Protein Characterization
- Protein Sciences
- Mammalian
- Microbial
- mRNA
- Viral Vaccines
- WuXi XDC – Bioconjugation
- Testing
- Sustainability
- Careers
- Investors
- News & Resources
WuXi Biologics
Offering End-to-End Solutions
WuXi XDC Overview

Your Single Source for Bioconjugation Discovery, Development and cGMP Manufacturing
Watch our video to learn more about the WuXi XDC integrated services advantage.
WuXi XDC, a WuXi Biologics subsidiary, and a leading Contract Research, Development and Manufacturing Organization (CRDMO), is a joint venture between WuXi Biologics and the WuXi AppTec subsidiary, WuXi STA. The joint venture greatly simplifies Antibody Drug Conjugate (ADC) and other bioconjugate drug development by providing all discovery, preclinical activities, CMC development and the entire manufacturing supply chain under one company and in one centralized region. WuXi XDC provides single-source development and manufacturing platforms for small molecule linkers / payloads and biologics (e.g., monoclonal, bispecific and multispecific antibodies or other recombinant proteins) intermediates with the company’s leading bioconjugation discovery, development and manufacturing services.
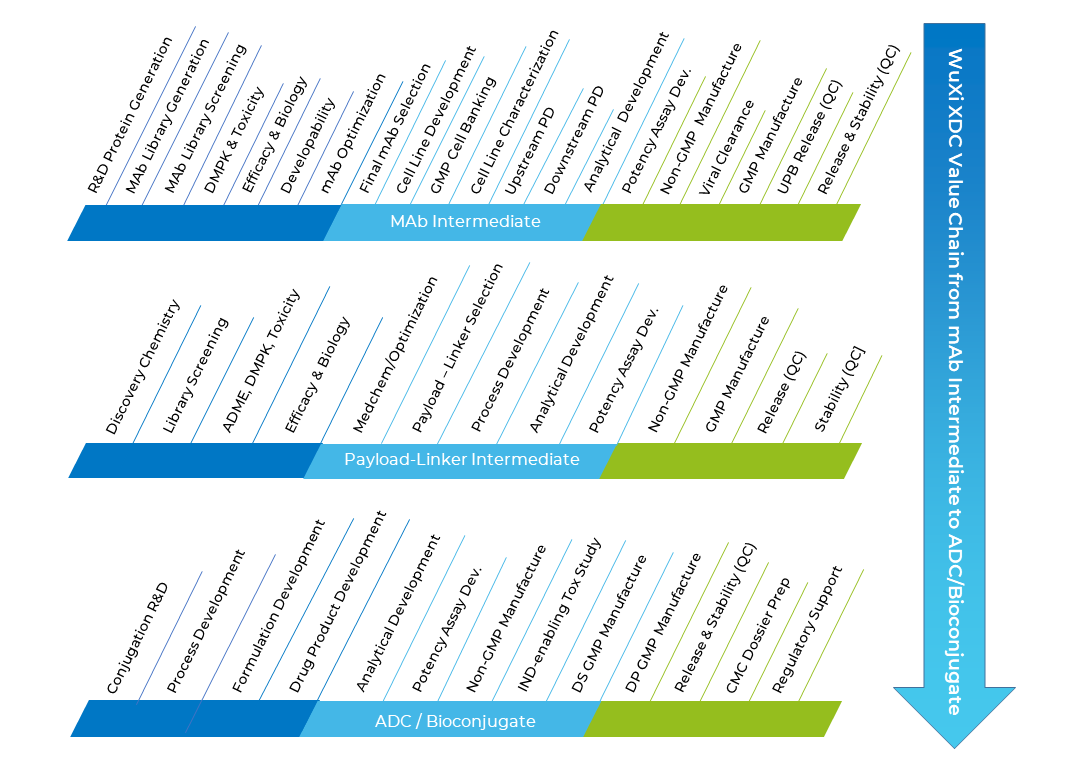
The WuXi XDC one-stop service and technology platform provides for our clients an unmatched value chain for the development of ADCs and other bioconjugates including conjugated diagnostic and imaging reagents.
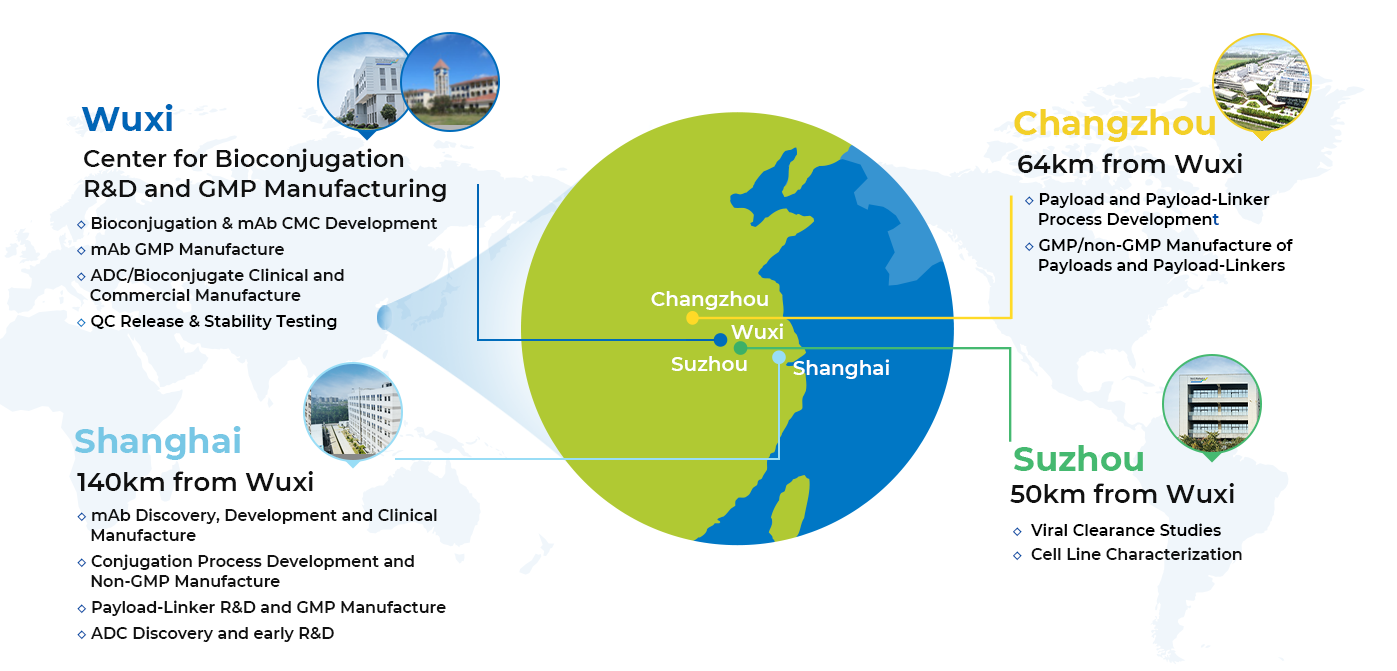
All activities and services performed at WuXi XDC are conducted within 1-2 hours (driving), thus providing an unprecedented geographic advantage.
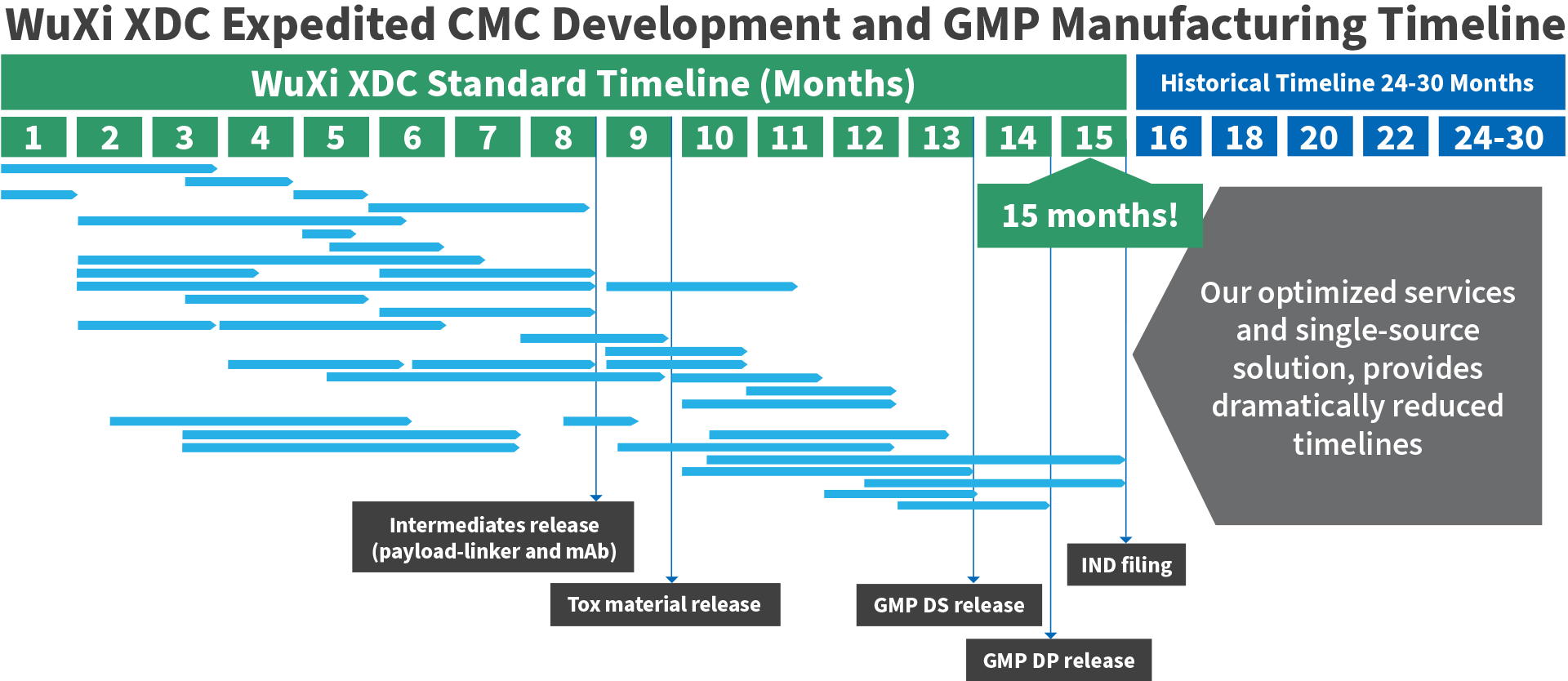
Our expertise, highly-efficient value chain and geographic advantage allows us to cut the traditional antibody drug conjugate CMC development period in half. We can start with cell line development activities for generation of the antibody intermediate and finish with an IND filing for the bioconjugate drug in only 15 months!
We provide world-class expertise in both platform and novel conjugation technologies and can handle highly potent and toxic payloads. Extensive payload and linker libraries as well as a variety of mAb library generation and screening platforms are available for development candidate matrix preparation and evaluation. A complete ADC analytical development toolbox can be leveraged to select the most appropriate analytical methods for specific bioconjugate characterization, and to establish lot release and stability assay panels. We offer a full range of services including both integrated mAb, payload-linker and bioconjugate development packages and standalone CMC development and drug substance (DS) and drug product (DP) manufacturing packages just for the bioconjugate or intermediates. We extend our extensive bioconjugation services for diagnostic and imaging reagents and we also provide “ready-made” GMP-grade chemical payloads / linkers, many with an existing Drug Master File (DMF).
>295Ongoing bioconjugate discovery projects |
>90Ongoing, integrated CMC development programs* |
>10Ongoing late-stage, integrated (Phase II / III) programs*
|
>40Successful INDs filed by our clients, enabled through WuXi XDC services |
*Integrated programs comprise product development and manufacturing programs contracted to us by our clients that utilize multiple WuXi XDC development capabilities / technologies or services (e.g., cell line development, process development, analytical development, formulation and drug product development etc.) and GMP manufacturing (drug substance, drug product or both)
We work with over 260 clients from around the globe throughout the bioconjugate discovery to late-stage development spectrum. Below are just a few of our clients ranging from venture-backed start-ups to large-pharma that we work with to bring novel ADCs and other bioconjugate drugs into the clinic and beyond for the benefit of patients worldwide.

For more information
White paper:
The Watershed Moment for ADCs has Arrived
Podcasts:
Antibody Drug Conjugate (ADC) Development And Manufacturing Challenges And Solutions
Webinar:
Antibody Drug Conjugates (ADC) drug development and manufacturing trends, challenges and solutions





















