- Services & Solutions
- Technologies
- Discovery
- Reagent Materials Generation
- Monoclonal Antibody Discovery
- Bispecific & Multispecific Antibody Generation
- Lead Optimization
- In Vitro Lead Characterization
- In Vivo Lead Characterization
- Other Molecular Modalities
- IND Filing Support
- Protein Sciences
- Antibody Production
- Protein Production
- Protein Characterization
- Protein Sciences
- Mammalian
- Microbial
- mRNA
- Viral Vaccines
- WuXi XDC – Bioconjugation
- Testing
- News & Resources
WuXi Biologics
Offering End-to-End Solutions
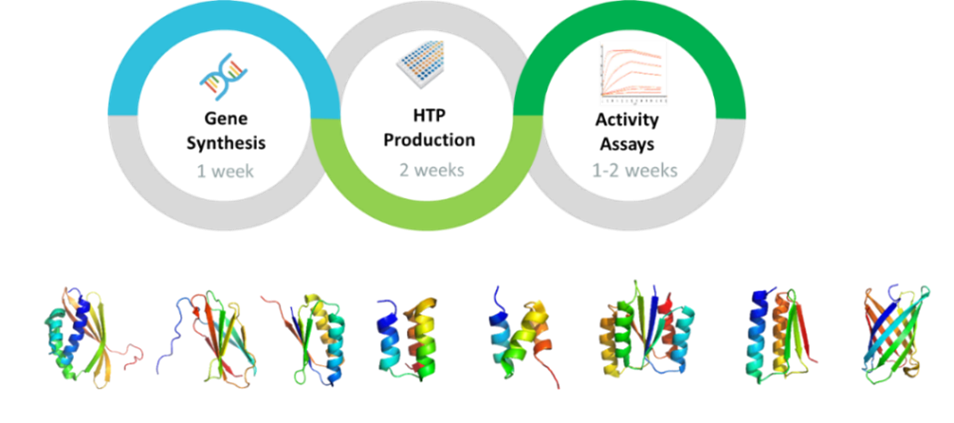
MiniProtein Line Service
High-Throughput, High-Titer Production of Miniproteins in CHO Cells
Miniproteins are de novo designed molecules, distinct from any found in nature. Typically, consisting of 100-200 amino acids, these miniproteins show various functions like efficient cell penetration due to the small size. Recently, there has been a surge of interest among artificial intelligence (AI) companies in creating miniproteins to pave the way for innovative therapeutics development.
Historically, miniproteins have been produced in E. coli, a method fraught with challenges such as limited throughput and high levels of endotoxins. To overcome these obstacles, we have developed our groundbreaking MiniProtein Line. This innovative platform leverages our high-titer CHO expression system, offering a much higher yield and substantially lower endotoxin levels compared to the conventional E. coli expression methods.
Furthermore, by integrating our high-throughput Bio-Layer Interferometry (BLI) services, we can swiftly determine the binding constants (Kd) of these miniproteins. This integration facilitates a rapid screening process, accelerating the development and analysis of the miniproteins.
Key Features of MiniProtein Line
- High-titer transient CHO expression, achieving exceptional yields ranging from 100 to 500 mg/L
- Capability for HTP production, coupled with rapid binding constant (Kd) measurements
- Streamlined process from sequence to binding assays, delivery within 4-5 weeks
MiniProtein Line Service Details:
| Service Item | Deliverables | Duration | QC | Request A Quote |
| 24 x 3 mL CHO | 0.5 mg, single-step affinity purification | 3-4 weeks | Bradford, SEC-HPLC, SDS-PAGE LC-MS (optional) | Request A Quote |
| ≥20 mL CHO | 2 mg from 20 mL, two-step purification | 3-4 weeks | SEC-HPLC, SDS-PAGE, endotoxin (<0.5 EU/mg) LC-MS (optional) |
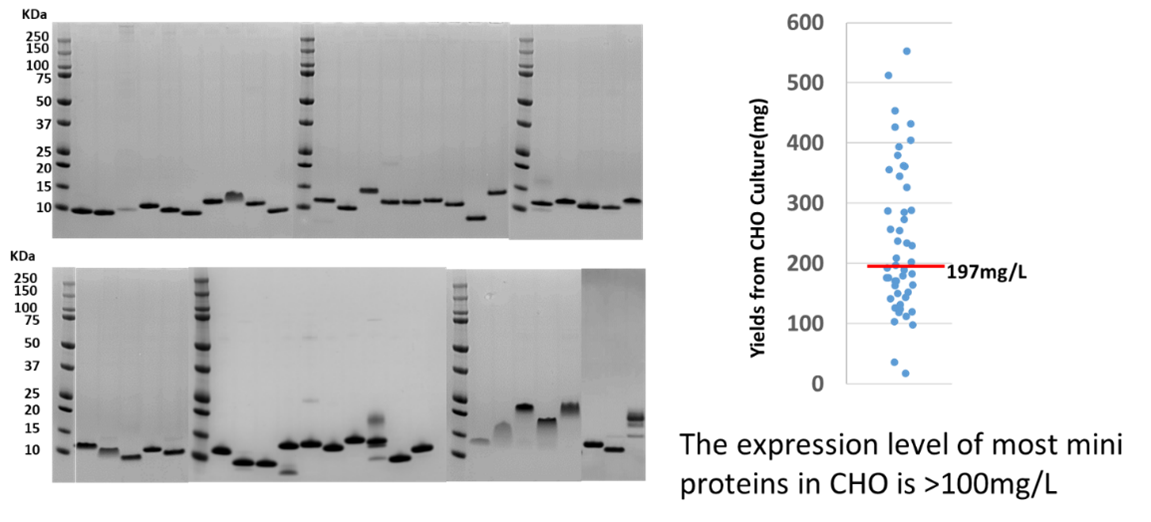
Case Study: Super High-Titer Expression of 48 Miniproteins with 20 Different Folds
In this case study, we demonstrated the exceptionally high-titer production of 48 miniproteins with 20 different folds (structures), using single-step purification via our MiniProtein Line service.
Figure A: The MiniProtein Line was utilized to produce 48 miniproteins, each with an N-terminal His-tag and purified using one-step Ni column purification. On average, the titer reached 197 mg/L, with the majority of miniproteins achieving a titer of 100 mg/L or more—a notable achievement for molecules of ~10 KDa in size.
Frequently Asked Questions for MiniProtein Line Service
Q: Is it possible to purify miniproteins without using a His-tag?
A: Absolutely. Besides His-tag, we also offer Flag-tag for miniproteins. But if your project is aiming for miniproteins with specific constructs, we can purify them without any tags, and then use conventional chromatography for purification.
Q: How do you check the purity of miniproteins?
A: We run SEC-HPLC to determine the purity of miniproteins. This technique separates the miniproteins based on their size, allowing us to pinpoint their relative abundance.
Q: How do you ensure the miniproteins are stable?
A: We’ve got various analytical methods to measure the stability of miniproteins. For instance, we can measure their melting temperature (TM) or examine their structure with circular dichroism (CD).
Q: Have you scaled miniprotein production beyond 1-liter scale?
A: Our Protein Sciences (PS) Department at WuXi Biologics focuses on the research domain, with our expertise primarily rooted in smaller-scale projects. However, when scaling up to 1 L using our CHO transient platform, we can achieve yields of 400-500 mg of protein. We are capable of scaling up further without an upper limit.
Q: Can you offer computational services to enhance protein properties, such as solubility?
A: We do not offer computational services for miniprotein property improvement. The field of miniproteins is rapidly advancing, and we lean on our client’s expertise to guide protein development.
Q: What types of downstream assays do you provide for miniproteins?
A: After producing miniproteins, our go-to downstream assays include BLI, SPR, and occasionally ELISA for certain cases. These methods, especially BLI, are highly efficient for HTP screening of miniproteins within a short timeframe. Additionally, we can conduct sophisticated, low-throughput assays for your specialized proteins.
Q: What are the typical yields you've observed for miniproteins, and how do you determine a 'normal' range for well-expressed ones?
A: We consider expression level >100 mg/L to be typical for most miniproteins. In a case study where we expressed 48 miniproteins in CHO cells, the average yield was 197 mg/L.
Q: How does sequence homology with different species like E. coli, mammals, and insects affect the expression of de novo synthesized miniproteins?
A: Our understanding of sequence homology across various species and its impact on miniprotein expression is limited, since these proteins are designed de novo. Generally, we observe higher expression levels in CHO compared to E. coli, regardless of sequence homology. Other factors such as the maturity of the design algorithm and the structural characteristics of the miniproteins also influence expression levels.
Q: What considerations are important for improving the yield of de novo designed miniproteins?
A: The miniproteins’ structures are crucial for their yields. Miniproteins with stable hydrophobic cores with proper folding are more likely to be expressed successfully in both E. coli and CHO. For miniproteins designed to function intracellularly with free cysteines, E. coli is often preferred. Conversely, miniproteins lacking free cysteines or those with disulfide bonds are better expressed in CHO. Additionally, when miniproteins serve as building blocks for mAbs or bsAbs, CHO is recommended to achieve optimal expression levels.
Q: Can miniproteins that are insoluble in E. coli achieve soluble expression in CHO cells?
A: Yes, we have encountered cases where miniproteins that are insoluble in E. coli were successfully expressed in a soluble form in CHO cells.
Q: What are the minimum and maximum lengths of miniproteins that you can produce, and do you offer services for sequence design?
A: Our expression system produces miniproteins with lengths between 80 and 200 amino acids. The constraint on the maximum size is primarily related to the capabilities of algorithmic design rather than our expression system. Currently, we do not provide services for sequence design.
Q: How does the pI range influence the selection of miniprotein sequences, and does it affect expression or purification efficiency?
A: While the pI range of miniproteins typically does not pose issues, miniproteins with a high concentration of basic amino acids can lead to proteolysis in both E. coli and CHO cells. Monitoring the proteolysis profile of these miniproteins is essential for in vivo therapeutic applications to ensure their stability and effectiveness. To support this, we offer serum stability tests as part of our micro developability services.
Q: Do you offer assays to evaluate the immunogenicity of miniproteins?
A: Yes, we offer both in silico immunogenicity prediction to identify sequences with potential immunogenicity issues and ex vivo immunogenicity assays to assess the immunogenicity risk of miniproteins.
Related Content
- Brochures: Featured Services for Protein & Antibody Production, E. coli Recombinant Protein Production
- Service Spotlight: Protein Characterization Services
- Blog: Read Our Blog for Insights in the World of Proteins







